A 6-item parent-completed survey designed to measure the broad impact of asthma on children.




 NEW COAS IN ONCOLOGY GUIDE
NEW COAS IN ONCOLOGY GUIDE
QM COAs can help capture slowed decline in physical functioning and well-being. Learn how to illuminate the impact of cancer treatments and therapies using COA measures in a free oncology-focused guide.
Read the Guide




If you need a translation in a specific language, consult with our translation team to determine a cost estimate and timeline that fits to your project needs.





Combining our eCOA Platform with research expertise, our consultants help your teams develop, validate, implement and analyze PROs with a custom approach. Our services ensure you gain the most meaningful insights on patient-reported data.





Our proprietary COA desktop software, which provides the ability to collect, score and interpret data for your study or program.
Learn MoreA customizable cloud-based data collection and reporting portal for capturing data from patients or clinicians securely and accurately.
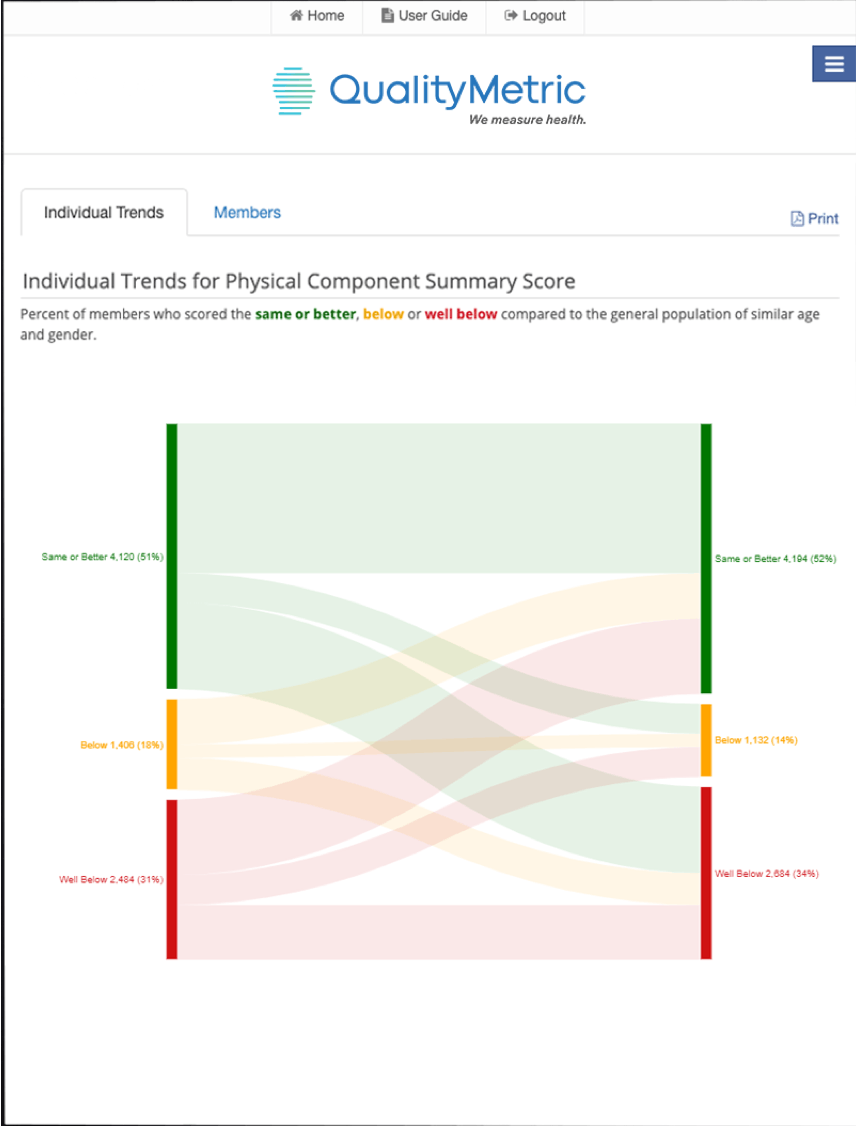
Learn MoreOur advanced reporting feature, built to seamlessly convert patient-reported data into reports with actionable insights.
Learn MoreStandardized solutions for scoring datasets with absolute accuracy and identifying errors that may impact interpretation of results.
Learn More