The healthcare industry has historically suffered from a low volume of valuable research and available treatments that address women’s health issues. One area particularly unexplored is premenstrual syndrome (PMS), and by extension, premenstrual dysphoric disorder (PMDD).
A majority of women of reproductive age experience physical or psychological discomfort surrounding the first few days in the menstruation phase of a regular menstrual cycle. This pain, which ranges from mild to extreme, affects a woman’s health-related quality of life (HRQoL), and although premenstrual symptoms may be tolerable for some, they can also be debilitating and even disabling.
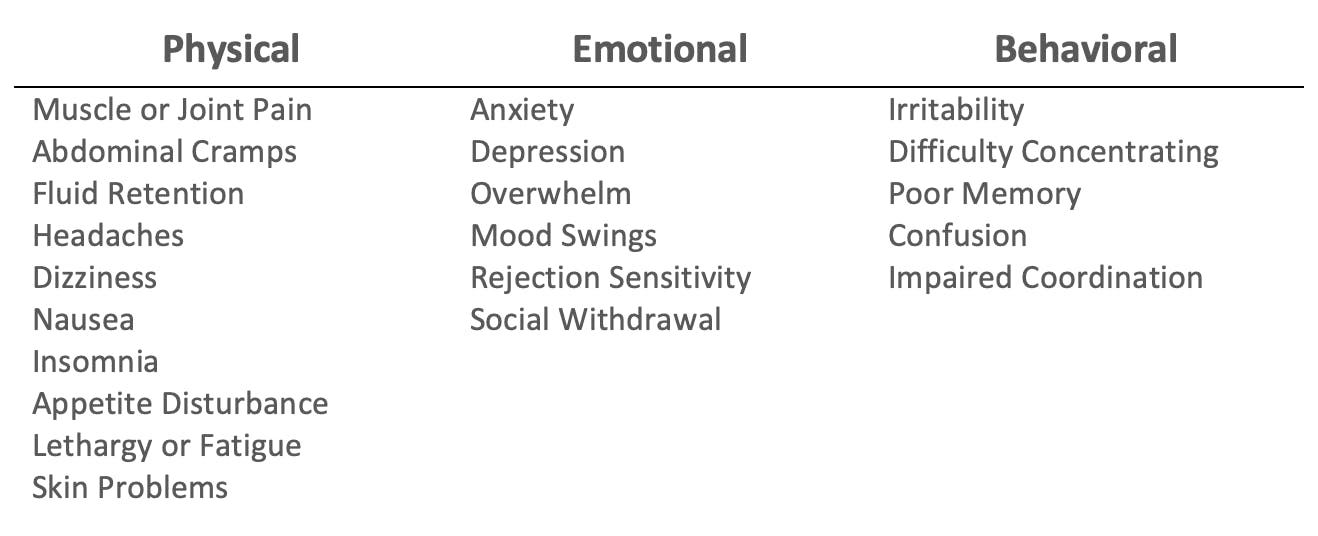
PMS describes a common pattern of physical, emotional and behavioral symptoms, while PMDD is distinguished by more severe premenstrual mood disturbances that may occur a week or more before menses and greatly impact social functioning. A recent study has also observed a reduction of quality-adjusted life years in those with PMDD.
Common Premenstrual Symptoms
The Power of PROs for PMS & PMDD
To drive innovation in a range of therapeutic areas, researchers and clinicians are increasingly utilizing patient-reported outcomes (PROs), a clinical outcome assessment (COA) tool that gathers data from the patient’s perspective to achieve a variety of research and care initiatives. Because both PMS and PMDD are characterized by premenstrual signs that span physical, emotional, cognitive and social domains, more healthcare professionals are recognizing the power of examining such symptoms through the lens of patients.
With a simple PRO premenstrual symptoms test, patients and clinical trial participants can answer straightforward questions regarding their conditions and experiences to equip professionals with real-world data that can inform everything from treatment decision-making to product development. PRO measures are known to offer a host of benefits for researchers, clinicians and patients alike, giving teams data-driven insights and patients an opportunity to feel seen, heard, listened to and educated regarding their personal care.
Measuring HRQoL with PMSIS
One patient-first premenstrual symptoms test that has proven effective for measuring HRQoL among women with PMS and PMDD is the Premenstrual Symptoms Impact Survey(PMSIS) by QualityMetric—a validated six-question PRO assessment administered to females aged 18 and older with PMS or PMDD. It is designed to quantify the functional health and well-being of women from their own point of view to determine the impact of premenstrual symptoms on physical, social and cognitive functioning domains over time, with diagnostic criteria that may also identify at-risk individuals. Interpreting PMSIS scores is simple, and survey administrators can obtain certified scoring assistance directly from QualityMetric scientists.
With the Premenstrual Symptoms Impact Survey, an award-winning short-form PRO health instrument, QualityMetric and community-owned health data platform courage healthcare teams around the world to collect patient-reported health data and lead smarter research studies that help revolutionize women’s health.
Contact QualityMetric to learn more about licensing the PMSIS or other health surveys for use in your own research or care.
One patient-first premenstrual symptoms test that has proven effective for measuring HRQoL among women with PMS and PMDD is the Premenstrual Symptoms Impact Survey(PMSIS) by QualityMetric1—a validated six-question PRO assessment administered to females aged 18 and older with PMS or PMDD.
References
- Wallenstein GV, Blaisdell-Gross B, Gajria K, et al. Development and validation of the Premenstrual Symptoms Impact Survey (PMSIS): a disease-specific quality of life assessment tool. J Womens Health (Larchmt). 2008;17(3):439-450. https://doi.org/10.1089/jwh.2007.0377
 NEW COAS IN ONCOLOGY GUIDE
NEW COAS IN ONCOLOGY GUIDE
